ICCBH2015 Poster Presentations (1) (201 abstracts)
Mineral metabolism in children with autosomal dominant polycystic kidney disease
Stéphanie De Rechter 1, , Justine Bacchetta 3 , Laurence Dubourg 3 , Pierre Cochat 3 , Mieke Van Dyck 1 , Pieter Evenepoel 4 , Elena Levtchenko 1, & Djalila Mekahli 1,
1Department of Pediatric Nephrology, Leuven, Belgium; 2Laboratory of Pediatrics, KU Leuven, Leuven, Belgium; 3Centre de Référence des Maladies Rénales Rares, Hospices Civils de Lyon, Bron, France; 4Department of Internal Medicine, Division of Nephrology, Leuven, Belgium.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic cause of renal failure. Data from adult ADPKD population show increased fibroblast growth factor 23 (FGF23) levels while circulating Klotho levels decrease, with a low TmP/GFR even in patients with normal renal function. Moreover, in ADPKD animal models, cyst lining renal cells were demonstrated to produce FGF23, although the animals displayed FGF23 resistance. No data are available in a paediatric ADPKD population. In this multicentre study, we prospectively assessed bone metabolism and renal phosphate handling in children with ADPKD and normal renal function by analysing blood and urine parameters. Based on normal values according to age, we made percentile (P) charts for serum phosphate and TmP/GFR. Hypophosphatemia and urinary phosphate wasting were defined as values ≤P5. We included 119 ADPKD patients (67 males, median (range) age 10.5 (0.5–18.6) years). PTH levels were in the normal range, 45% of children displayed vitamin D deficiency. Hypophosphatemia and urinary phosphate wasting were observed in 11, and 27% of the children respectively (Figure 1). This is the first report highlighting hypophosphatemia in combination with renal phosphate leak in ADPKD children with normal eGFR. Our results confirm recent data in adult ADPKD patients and point to an abnormal FGF23 metabolism starting at paediatric age. Further studies are required to elucidate the underlying pathophysiology and to investigate potential clinical consequences.
Stéphanie De Rechter, Djalila Mekahli and Elena Levtchenko are supported by the Fund for Scientific Research, Flanders.
Disclosure: The authors declared no competing interests.
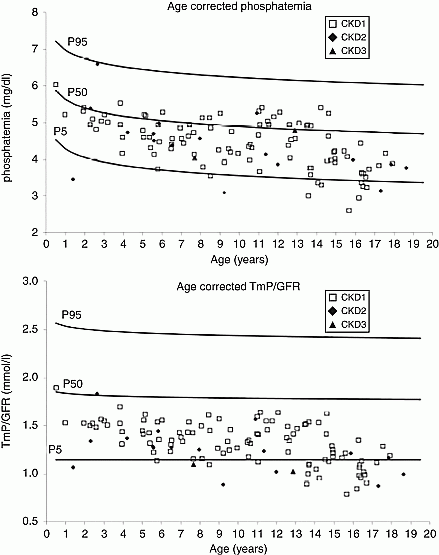
Figure 1




